
The future seems bright for hair loss sufferers, there are two up and coming products that may revolutionize the hair loss industry. Breezula (clascaterone) has surfaced as a topical anti-androgen that on paper may be more effective than Propecia (finasteride).
Replicel (RCH-01) is being touted as a hair loss cure. A revolutionary treatment that may put an end to balding once and for all.
In this article, we will be discussing Replicel (RCH-01) as a future treatment for genetic hair loss.
What is Replicel (RCH-01)
Replicel (RCH-01) is an autologous cell therapy that uses dermal sheath cup (DSC) cells that are isolated from the hair follicle to treat androgenic alopecia (genetic hair loss). I know this may sound very technical and confusing, but allow us to explain in plain English.
Dermal sheath cup (DSC) cells are located at the base of the hair follicle. These cells regulate the growth of hair fibers in the hair follicle. Those who suffer from male pattern baldness have vulnerable cells in their hair follicle receptor sites. These vulnerable cells are attacked by the hormone DHT.
The hormone DHT eventually degrades the hair follicle until it no longer grows. However, the DSC cells on the back and sides of the scalp are immune to DHT, this is why hereditary hair loss follows a distinct pattern according to RepliCel Life Sciences.
So How Will Replicel (RCH-01) Cure Hair Loss?
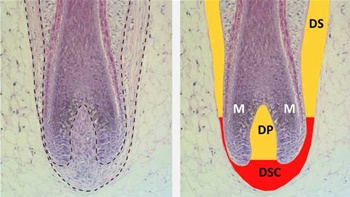
Replicel (RCH-01) will be made up of DSC cells that are immune to the hormone DHT. These cells will be harvested from a small tissue sample taken from the back of the patient's scalp. The tissue will be used to grow millions of these immune cells, which will then be reintroduced into the patient's balding area. Thus, curing hair loss as we know it.
To date, Replicel (RCH-01) is under clinical investigation at Tokyo Medical University Hospital and Toho University Ohashi Medical Center, by Dr. Tsuboi and Dr. Niiyama. The study is being financed by the Shisheido Company and each product will be manufactured and distributed by Shisheido at their Cell-Processing and Expansion Center.
What Do The Clinical Studies Say About Replicel (RCH-01)
In 2017, phase 1 of the human clinical trial for Replicel (RCH-01) was completed. The study showed the product was safe for consumers and provided some promising results for the treatment of genetic hair loss. A recent study was completed in Tokyo Medical University Hospital and Toho University Ohashi Medical Center in Japan.
The clinical data from this study may be enough for Shisheido the partners of Replicel Life Sciences to move forward with distribution in Asia. However, the decision to launch the product in Asia will be the sole decision of Shisheido.
Bottomline
While the words "hair loss cure" sound exciting the truth is very little is known about Replicel (RCH-01). At this point, Replicel (RCH-01) is nothing more than a twinkle in a hair loss sufferers eye. While yes, phase 1 of human clinical trials have been completed and reportedly show promise.
In most countries, it takes a minimum of three-phase clinical trials to obtain approval to market a product. However, Japan has recently created rules allowing certain cell therapies to launch much faster in comparison to other countries. This has led to some speculation that Replicel RCH-01 may be available by 2020 in Japan. However, if history is any indicator that will not be the case.

